MALACAÑAN PALACE
MANILA
BY THE PRESIDENT OF THE PHILIPPINES
[ EXECUTIVE ORDER NO. 106, February 26, 2020 ]
PROHIBITING THE MANUFACTURE, DISTRIBUTION, MARKETING AND SALE OF UNREGISTERED AND/OR ADULTERATED ELECTRONIC NICOTINE/NON-NICOTiNE DELIVERY SYSTEMS, HEATED TOBACCO PRODUCTS AND OTHER NOVEL TOBACCO PRODUCTS, AMENDING EXECUTIVE ORDER NO. 26 (S. 2017) AND FOR OTHER PURPOSES
WHEREAS, Article II, Sections 15 and 16 of the Constitution mandates the State to protect and promote the right to health of the people and instill health consciousness among them, as wel! as protect and advance the right of the people to a balanced and healthful ecology in accord with the rhythm and harmony of nature;
WHEREAS, the World Health Organization (WHO) issued a report in August 2016 on Electronic Nicotine and Non-Nicotine Delivery Systems (ENDS/ENNDS), which noted that (i) the use of adulterated and even unadulterated ENDS/ENNDS produces aerosol that ordinarily includes toxicants which trigger a range of significant pathological changes, and (ii) ENDS/ENNDS are unlikely to be harmless, such that long-term use is expected to increase the risk of chronic obstructive pulmonary disease, lung cancer, possible cardiovascuiar disease, as well as some other diseases associated with smoking;
WHEREAS, Republic Act (RA) No. 11467 mandates the Food and Drug Administration (FDA) to periodically determine and regulate, consistent with evolving medical and scientific studies, the manufacture, importation, sale, packaging, advertising and distribution of heated tobacco products (HTPs) and ENDS/ENNDS, including banning the sale to persons below twenty-one (21) years old;
WHEREAS, RA No. 7394 or the "Consumer Act of the Philippines," declares it a policy of the State to ensure safe and good quality of food, drugs, cosmetics and devices, and regulate their production, sale, distribution and advertisement, to protect the health of consumers, and designates the Department of Health (DOH), through the FDA, as implementing agency therefor;
WHEREAS, RA No. 7394 prohibits the adulteration of any food, drug : device, or cosmetic and the manufacture, importation, exportation, sale, offering for sale, distribution or transfer of any food, drug, device or cosmetic that is adulterated;
WHEREAS, RA No. 9711 or the "FDA Act of 2009," provides that the State shall adopt, support, establish, institutionalize, improve and maintain structures, processes, mechanisms and initiatives that are aimed, directed and designed to protect and promote the people's right to health;
WHEREAS, RA No. 9711 also prohibits the adulteration of health products, and the manufacture, importation, exportation, sale, offering for sale, distribution, transfer, non-consumer use, promotion, advertising or sponsorship of any health product that is adulterated;
WHEREAS, RA No. 8749 or the "Philippine Clean Air Act of 1999," defines air pollutant as any matter found in the atmosphere other than the inert gases in their natural or normal concentrations, that is detrimental to health or the environment, which includes but is not limited to smoke, dust, soot, cinders, fly ash, solid particles of any kind, gases, fumes, chemical mists, steam and radioactive substances;
WHEREAS, Executive Order (EO) No. 26 (s. 2017) prohibits smoking in certain public places, provides for the establishment of designated smoking areas, restricts access to tobacco products especially by minors, and imposes requirements and limitations on the advertising and promotion thereof;
WHEREAS, the use of ENDS/ENNDS, HTPs and other novel tobacco products, as a shift from conventional lighted tobacco products, has proliferated in the country;
WHEREAS, the DOH has reported that users and bystanders exposed to emissions from the use of ENDS/ENNDS, HTPs and other novel tobacco products are at similar risk of respiratory illness, cardiovascular diseases, addiction, cancer, neurodegeneration, brain development retardation, anxiety, and sexual and reproductive dysfunctions, among other health conditions;
WHEREAS, there is a need to regulate access to and use of ENDS/ENNDS, HTPs and other novel tobacco products, to address the serious and irreversible threat to public health, prevent the initiation of non-smokers and the youth, and minimize health risks to both users and other parties exposed to emissions;
WHEREAS, Article VII, Section 17 of the Constitution vests the President with the power of control of all the executive departments, bureaus and offices, and mandates him to ensure that the laws be faithfully executed;
NOW, THEREFORE, I, RODRIGO ROA DUTERTE, President of the Philippines, by virtue of the powers vested in me by the Constitution and existing laws, do hereby order:
Section 1. Prohibition of Unregistered and/or Adulterated ENDS/ENNDS, HTPs and Other Novel Tobacco Products. The manufacture, distribution, marketing or sale of unregistered or adulterated ENDS/ENNDS, components thereof in the form of devices, e-liquids, solutions or refills whether physically part of or intended to be used with ENDS/ENNDS, HTPs and other novel tobacco products, are hereby prohibited and shall be dealt with in accordance with this Order and existing laws.
Section 2. Regulatory Framework for ENDS/ENNDS, HTPs and Other Novel Tobacco Products. All e-liquids, solutions or refills forming components of ENDS/ ENNDS or HTPs shall be registered with the FDA, in accordance with RA Nos. 9711 and 11467.
All devices forming components of ENDS/ENNDS or HTPs shall be subject to the product standards imposed by the Department of Trade and Industry and the FDA, in accordance with RA Nos. 7394 and 11467.
Other novel tobacco products shall be regulated in accordance with RA No. 9211 and other relevant issuances, and are subject to the jurisdiction of the Inter-Agency Committee-Tobacco (IAC-T), established under the said law.
Section 3. License to Operate. All establishments engaged in the manufacture, distribution, importation, marketing and sale of ENDS/ENNDS, HTPs, or their components, shall secure a License to Operate (LTO) from the FDA.
Section 4. Importation. The entry/importation of unregistered or adulterated ENDS/ENNDS, HTPs, or components thereof is hereby prohibited. For this purpose, the FDA and DTI are hereby directed to coordinate with the Bureau of Customs in the formulation of the guidelines, requirements and procedures for the regulation of the entry/ importation of ENDS/ENNDS, HTPs, and their components into the Philippine market.
Section 5. Implementing Guidelines. Within thirty (30) days from the effectivity of this Order, the FDA, in consultation with relevant agencies and stakeholders, shall formulate and issue the rules, regulations and standards governing the registration of ENDS/ENNDS, HTPs and their components, and the issuance of LTOs in relation thereto, required in this Order.
Section 6. Expansion of the Coverage of EO No. 26. Section 1 of EO No. 26, is hereby amended as follows:
"Section 1. Definition. For the purpose of this Executive Order, the following terms shall mean:
(a) "Advertising and promotion" means any form of commercial communication, recommendation or action with the aim, effect or likely effect of promoting tobacco products, ENDS/ENNDS, HTPs, or their components, or the use thereof, either directly or indirectly.
(b) "Designated Smoking/Vaping Area" (DSVA) refers to an area of a building or conveyance where smoking and vaping may be allowed, which may be in an open space or separate area with proper ventilation subject to the specific standards provided in this Order.
xxx
(e) "Non-Smoking/Vaping Buffer Zone "(Buffer Zone) is a ventilated area between the door of a DSVA not located in an open space and the smoke/vape-free area. There shall be no opening that will allow air to escape from such Non-Smoking/Vaping Buffer Zone to the smoke/vape-free area, except for a single door equipped with an automatic door closer. Such door is distinct from the door of the DSVA , which shall be at least two (2) meters away from the other.
xxx
(h) "Point-of-sale" refers to any location at which an individual can purchase or otherwise obtain tobacco products, ENDS/ENNDS, HTPs, or their components.
xxx
(k) "Smoke/vape-free" refers to air that is 100% free from smoke, vapor or aerosol from tobacco products, ENDS/ENNDS or HTPs. This definition includes, but is not limited to, air in which such smoke, vapor or aerosol cannot be seen, smelled, sensed or measured.
(I) "Smoking/vaping" means being in possession or control of a tobacco product or a powered ENDS/ENNDS or HTP, regardless of whether the emission in the form of smoke, vapor or aerosol is being actively inhaled or exhaled.
(m) Tobacco products" means products entirely or partly made of tobacco leaf as raw material which are manufactured to be used for smoking, sucking, chewing or snuffing, such as but not limited to cigarette, cigar, pipe, shisha/hookah and chew tobacco. The term shall exclude ENDS/ENNDS and HTPs and include other novel tobacco products.
xxx
(o) "Electronic Nicotine/Non-Nicotine Delivery Systems" (ENDS/ ENNDS), otherwise known as electronic cigarettes or vapes, are e-liquids, solutions or refills, whether or not containing nicotine, and an electronic delivery device, or any combination thereof, that produce an aerosol, mist or vapor that users inhale by mimicking the act of smoking. ENDS/ENNDS deliver nicotine and/or other chemicals to the lungs after one end of a plastic or metal cylinder is placed in the mouth, like a cigarette or cigar, and inhaled to draw a mixture of air and vapors from the device into the respiratory system. They contain electronic vaporization systems, rechargeable batteries and chargers, electronic controls and replaceable cartridges containing nicotine and/or other chemicals. For the avoidance of doubt, the term ENDS/ENNDS is coextensive with the term "vapor products" as defined in RA No. 11467.
(p) "Heated Tobacco Product" (HTP) refers to a product that may be consumed through heating tobacco, either electrically or through other means, sufficient to release an aerosol that can be inhaled, without burning or combustion of the tobacco. HTPs include liquid solutions and gels that are part of the product and are heated to generate an aerosol.
(q) "Novel tobacco products" refers to all substances, devices and innovations entirely or partly made of tobacco leaf as raw material, already existing or to be developed in the future, intended to be used as substitutes for cigarettes, conventional tobacco products, ENDS/ENNDS or HTPs.
Section 7. Expansion of Prohibited Acts under EO No. 26. Section 3 of EO No. 26 is also amended as follows:
"Section 3. Prohibited Acts. The following acts are declared unlawful and prohibited:
(a) Smoking/vaping within enclosed public places and public conveyances, whether stationary or in motion, except in DSVAs fully compliant with the requirements of Section 4 of this Order;
(b) For persons-in-charge to allow, abet or tolerate smoking/vaping in places enumerated in the preceding paragraph, outside of DSVAs fully compliant with Section 4 of this Order;
(c) For any person to sell, distribute or purchase tobacco products to or from minors, or ENDS/ENNDS, HTPs, or their components to or from persons below twenty-one (21) years old. It shall not be a defense for the person selling, distributing or purchasing that he/she did not know or was not aware of the real age of the person he/she is transacting with. Neither shall it be a defense that he/she did not know nor had any reason to believe that the cigarette or any other tobacco product, ENDS/ENNDS, HTPs, or their components, was for the consumption of the person who received it;
(d) Use, sale or purchase of cigarettes or other tobacco products by a minor, or of ENDS/ENNDS, HTPs, or their components by a person below twenty-one (21) years old;
(e) Ordering, instructing or compelling the use, lighting up, purchase, sale, distribution, delivery, advertisement or promotion of tobacco products by a minor, or of ENDS/ENNDS, HTPs, or their components by a person below twenty-one (21) years old;
(f) Selling or distributing tobacco products, ENDS/ENNDS, HTPs, or their components in a school, public playground, youth hostels, recreational facilities for minors, areas frequented by minors, or within 100 meters from any point of the perimeter of these places;
(g) Placing, posting, displaying or distributing advertisement and promotional materials of tobacco products, ENDS/ENNDS, HTPs, or their components, such as but not limited to leaflets, posters, display structures and other materials in areas where their sale and distribution is prohibited;
(h) Placing any form of advertisement of tobacco products, ENDS/ ENNDS, HTPs, or their components outside the premises of point-of-sale retail establishments;
(i) Placing any stall, booth, and other displays promoting tobacco products, ENDS/ENNDS, HTPs, or their components, in areas outside the premises of point-of-sale locations or adult-only facilities:
(j) Failure to mark containers and packages of ENDS/ENNDS, HTPs, and the components thereof, with appropriate health warnings, pursuant to the content, format and specifications designated by the FDA, based on the actual ingredients or components of the product;
(k) Incorporating e-liquids, solutions and refills with flavors and additives that are proven or suspected to be appealing or enticing to persons below twenty-one (21) years of age, toxic, harmful, addictive or sensitizing; and
(l) Adding Tetrahydrocannabinol (THC) or cannabinoid compounds in liquids used in ENDS/ENNDS and HTPs. Violation of this provision shall be punishable in accordance with the applicable penalties provided under RA No. 9165 or the "Comprehensive Dangerous Drugs Act of 2002."
Section 8. Amending the Standards for Designated Smoking Areas. Section 4 of E.O. No. 26 is likewise amended as foilows:
"Section 4. Standards for DSVAs. All DSVAs shall strictly comply with the following standards:
(1) There shall be no opening that will allow air to escape from the DSVA to the smoke/vape-free area of the building or conveyance, except for a single door equipped with an automatic door closer; provided that, if the DSVA is not located in an open space, such door shall open directly towards a Buffer Zone as defined in this Order;
(2) The DSVA shall not be located in or within ten (10) meters from entrances, exits, or any place where people pass or congregate, or in front of air intake ducts;
(3) The combined area of the DSVA and the Buffer Zone shall not be larger than 20% of the total floor area of the building or conveyance, provided that in no case shall such area be less than ten (10) square meters;
(4) No building or conveyance shall have more than one DSVA; provided that persons-in-charge have the option of establishing one Designated Smoking Area and one Designated Vaping Area therein, subject to the same standards under this Section, with a combined area not exceeding 20% of the total floor area of the building or conveyance;
(5) The ventilation system for the DSVA other than in an open space and for the Buffer Zone shall be independent of ait ventilation systems servicing the rest of the building or conveyance;
(6) Minors shall not be allowed inside the DSVA and the Buffer Zone;
(7) The DSVA shall have the following signages highly visible and prominentiy displayed:
(7.1) "Smoking/Vaping Area signage;
(7.2) Graphic health warnings on the effects of using tobacco products, ENDS/ENNDS and HTPs; and
(7.3) Prohibition on the entry of persons beiow eighteen (18) years old.
(8) Other standards and specifications to better ensure a smoke/vape- free environment as may be prescribed by the IAC-T under RA No. 9211 and the FDA, provided that such standards and specifications are consistent with this Order and that persons-in-charge are given (60) days to comply.
However, there shall be no DSVAs in the following public places:
xxx
Nothing in this Order shall compel persons-in-charge to establish DSVAs nor prevent them from instituting more stringent measures in their buildings and establishments to better ensure a smoke/vape-free environment in their premises."
Section 9. Expanding the Duties of Persons-in-Charge. Section 5 of EO No. 26, is also amended as follows:
"Section 5. Duties and Obligations of Persons-in-Charge. Persons-in-charge shall:
(a) Prominently post and display the "No Smoking/Vaping" signage, in locations most visible to the public in the areas where smoking/ vaping is prohibited. At the very least, the "No Smoking/Vaping" signage must be posted at the entrance to the area, which shall be at least 8x11 inches in size, where the symbol shall occupy 60% of the signage, while the remaining 40% of the signage shall show the pertinent information/precautionary statement, as follows:
For example:
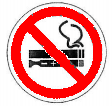
STRICTLY NO SMOKING/VAPING
Per EO No. 26 (s. 2017), as amended
Violators can be fined up to ________
Report violations to [hotline number]
As for the DSVA, after complying with the specifications in Section 4, prominently display the following elements in the signage:
"DESIGNATED SMOKING/VAPING AREA" or
"SMOKING/VAPING AREA"
[Place graphic/picture-based health warning on the effects of tobacco,
ENDS/ENNDS or HTP use within the signage]
[If available, place number of smoking/vaping cessation hotline]
(b) Prominently post and display the "No Smoking/Vaping" signage in the most conspicuous location within the public conveyance. At the very least, a three end a half (3.5) square inch "No Smoking/ Vaping" signage shall be placed on the windshield and a ten (10) square inch "No Smoking/Vaping" sign at the driver's back seat;
(c) Remove from the places where smoking/vaping is prohibited all ashtrays and other receptacles for disposing cigarette/electronic cigarette refuse;
(d) For persons-in-charge of schools, public playgrounds, youth hostels and recreational facilities for minors, including those frequented by minors, post the following statement in a clear and conspicuous manner:
SELLING, ADVERTISING AND PROMOTING CIGARETTES,
ELECTRONIC NICOTINE/NON-NICOTINE DELIVERY SYSTEMS,
HEATED TOBACCO PRODUCTS OR OTHER TOBACCO
PRODUCTS NOT ALLOWED WITHIN 100 METERS FROM ANY
POINT IN THE PERIMETER OF [NAME OF SCHOOL /
PLAYGROUND / FACILITY FOR MINORS / ETC.]
(e) For persons-in-charge of schools, public playgrounds, youth hostels and recreational facilities for minors, including those frequented by minors, to report to the nearest Smoke/Vape-Free Task Force of the concerned city or municipality any sale, advertisement or promotion of tobacco products, ENDS/ENNDS, HTPs, or their components, located within 100 meters from its perimeter;
(f) For persons-in-charge of point-of-sale establishments, post the following notice, together with a graphic/picture-based health warning on the health consequences of tobacco product, ENDS/ ENNDS or HTP use as prescribed by the DOH, in a clear and conspicuous manner:
SALE/DISTRIBUTION OF TOBACCO PRODUCTS TO MINORS,
OR ELECTRONIC NICOTINE/NON-NICOTINE DELIVERY
SYSTEMS OR HEATED TOBACCO PRODUCTS TO PERSONS
BELOW 21 YEARS OLD, IS UNLAWFUL
(g) Establish internal procedure and measures through which this Ordershall be implemented and enforced within the area of which he or she is in charge. This includes compliance with smoking/vaping, sales, distribution, advertising and promotions restrictions (e.g., warning smoking/vaping violators in banned areas and requesting them to stop smoking/vaping or leave the premises), and if they still refuse to comply, reporting the incident to the City/Municipal Health Office, the nearest peace officer, or to any member of the Smoke/Vape-Free Task Force;
xxx"
Section 10. Expansion of the Smoking Cessation Program. Section 8 of EO No. 26 is also further amended as follows:
"Section 8. Smoking Cessation Program. Local Government Units (LGUs), particularly the respective City/Municipai Health Officer, in coordination with the DOH, are enjoined to develop, promote and implement their respective Local Smoking Cessation Programs consistent with the National Smoking Cessation Program established pursuant to RA No. 9211, and to encourage the participation of public and private facilities which may be able to provide for the requirements of the program. Such Programs should include the implementation of the provisions of this Order regarding ENDS/ENNDS, HTPs and other novel tobacco products, Smokers/vapers who are willing to quit and/or those found violating this Order may be referred to the said Program and its facilities."
Section 10. Expansion of the Mandate of the Smoke-Free Task Force. Section 9 of E.O. No. 26 is likewise amended as follows:
"Section 9. Smoke/Vape-Free Task Force. Ail cities and municipalities are enjoined to form a local Smoke/Vape-Free Task Force to help carry out the provisions of this Order. Members of the Philippine National Police and Smoke/Vape-Free Task Forces are directed to carry out the provisions of this Order, including the apprehension of violators and the institution of criminal proceedings for violations of this Order, in accordance with relevant laws, rules and regulations, and strictly observing due process.''
Section 12. Separability. If any part of this Order is held unconstitutional or invalid, the other provisions not otherwise affected shall remain in full force and effect.
Section 13. Repeal. Except for the foregoing amendments, all other provisions of EO No. 26 shall remain unchanged and in effect. All rules, regulations and issuances or parts thereof which are inconsistent with this Order are hereby revoked, amended or modified accordingly.
Section 14. Effectivity. This Order shall take effect fifteen (15) days after publication in a newspaper of general circulation.
DONE, in the City of Manila, this 26th day of February in the year of our Lord. Two Thousand and Twenty.
(SGD.) RODRIGO ROA DUTERTE
By the President:
(SGD.) SALVADOR C. MEDIALDEA
Executive Secretary
The Lawphil Project - Arellano Law Foundation

|



